Key Takeaways
- Your glucose patterns may signal metabolic stress that influences your lung health.
- Insulin resistance, inflammation, and metabolic syndrome are linked to reduced lung function.
- Signos offers insight to monitor glucose trends and respond with targeted lifestyle tweaks, so you can support your metabolism and your lungs.
that {{mid-cta}}
Your lungs and metabolic health are more connected than you might realize. While we often consider glucose metabolism and insulin resistance in the context of diabetes and weight management, recent public health research suggests that these metabolic markers also have significant relationships with respiratory diseases, oxidative stress, and inflammation.
In other words, your glucose data can reveal clues not just about blood-sugar regulation, but also about how well your lungs are functioning.
In this article, we explore how metabolic stress may influence lung structure and function, how CGM patterns could provide early warning signs, and what lifestyle strategies support both metabolism and respiratory function.
How Metabolism Impacts Lung Function

We don’t usually think about our metabolism and lung health, but the two may be more connected than most people realize. Insulin resistance — when your cells become less responsive to insulin — may also affect how well your lungs work. Even subtle changes in insulin sensitivity have been linked to lower lung capacity and reduced respiratory function.
Research from Turkey found that people with lower forced expiratory volume (FEV1) to forced vital capacity (FVC) ratios (a measure of how efficiently the lungs move air) were more likely to have insulin resistance. And among people with impaired lung function from asthma, those with moderate to severe insulin resistance tend to lose lung function faster and respond less effectively to treatment.
Scientists believe this connection stems from inflammation and oxidative stress. When insulin resistance develops, it can spark low-grade, ongoing inflammation and increase oxidative damage throughout the body. It can also disrupt blood vessel health and energy metabolism, which can weaken the respiratory muscles and affect the cells that line the airways and lungs.
But insulin resistance is only part of the story. Metabolic syndrome (the cluster of factors like abdominal fat, high blood pressure, elevated glucose, and abnormal lipid metabolism of cholesterol or triglycerides) is also linked to poorer pulmonary function and reduced breathing capacity.
Even for people living with chronic obstructive pulmonary disease (COPD), one of the most common chronic lung diseases, insulin resistance often appears early, sometimes before symptoms worsen. This suggests that metabolic dysfunction may begin well before a noticeable decline in the function of the lungs.
Subtle Glucose Patterns as Early Clues
While standard fasting glucose and HbA1c tests are essential, continuous glucose monitors (CGMs) may help identify patterns in post-meal spikes or prolonged elevated blood glucose that could indicate early metabolic stress, even in individuals without type 2 diabetes.
One study found that glucose trends tracked by a CGM predicted lung function decline in people with cystic fibrosis, while the 2-hour oral glucose tolerance test (OGTT) didn’t. While this research focused on a very specific group, it suggests that fluctuations in blood sugar may be an early indicator of metabolic strain that affects respiratory function.
Elevated or variable nighttime glucose levels could also activate the body’s stress response, disrupt restorative sleep, and may contribute to subtle breathing changes or respiratory muscle fatigue, especially in those with obstructive lung disease or sleep-related breathing abnormalities.
In other words, glucose tracking can offer valuable insights into metabolic patterns that may not be apparent on standard lab tests but could be important predictors of lung disease risk.
Recognizing Risk Factors

Everyday habits, body composition, and even your environment can shape how well your metabolism and respiratory system work together. Here are some key risk factors to consider:
- Body Composition: Excess body fat, especially visceral fat around the abdomen, can increase the risk of insulin resistance, chronic inflammation, and even restrict lung expansion by putting extra pressure on the diaphragm and chest wall. In one study, waist circumference was identified as the strongest component of metabolic syndrome linked to reduced lung function.
- Lifestyle Habits: Diets high in refined carbohydrates, saturated fats, and low in fiber, along with prolonged sitting or inactivity, are linked to an increased risk of metabolic and inflammatory stress.
- Environmental Exposures: Cigarette smoke, air pollution, and even certain workplace exposures all contribute to inflammation that can lead to lung damage. Research suggests that the link works both ways: reduced airflow may exacerbate insulin resistance, and insulin resistance can impair the lungs' ability to function.
- Genetic Factors: Some people may be more predisposed to issues that affect both insulin signaling and lung function. Early metabolic stress can make lungs more vulnerable, while existing lung conditions can increase metabolic strain on the body.
Lifestyle Interventions That Support Lungs and Metabolism

Small, consistent changes in how you eat, move, sleep, and manage stress can reduce inflammation, improve insulin sensitivity, and protect your respiratory system over time.
Nutrition: Focus on Anti-Inflammatory, High-Fiber Meals
Mediterranean-style eating patterns have been linked to improved outcomes for both glucose homeostasis and lung function. Choose whole foods, such as legumes, whole grains, fruits, and vegetables. You can also include healthy fats from sources like olive oil or fatty fish that are rich in fatty acids and antioxidants.
Limiting refined carbs and added sugars helps reduce glucose spikes that may add to oxidative stress. Diets rich in fiber (at least 25 grams per day) also nourish the gut microbiome, which has a positive influence on inflammation.
Movement: Keep Your Body (and Lungs) in Motion
Regular movement is a powerful way to improve both insulin sensitivity and respiratory strength., Aerobic exercise, such as walking, swimming, or cycling, helps improve insulin sensitivity and boosts lung capacity and airflow.
Adding breathing-based movement (like yoga or diaphragmatic breathing) can also support lung function. And don’t underestimate the benefits of taking small breaks during the day: standing or stretching every hour can help balance the negative effects of prolonged sitting.
Sleep: Give Your Body the Time to Repair
Most adults need 7 to 9 hours of sleep per night, but consistency is just as important as duration. Poor or fragmented sleep can worsen insulin resistance and increase the risk of type 2 diabetes and cardiovascular disease.
It’s not always easy to fix poor sleep patterns, but you can start by making sure you have a cool, quiet, dark sleep environment paired with a relaxing wind-down routine. If you notice snoring or other breathing disturbances, it may be worth consulting a healthcare provider.
Stress: Calm Your Metabolism to Protect Your Lungs
Chronically elevated stress hormones like cortisol can raise blood glucose levels, increase insulin resistance, and trigger inflammatory enzymes and cytokines that can impact vascular and lung function. Simple practices such as mindfulness, meditation, or even a few deep breaths can help reset your nervous system. Managing stress not only improves sleep and mood but also supports healthier glucose patterns and better respiratory health.
How Signos Helps You Connect the Dots
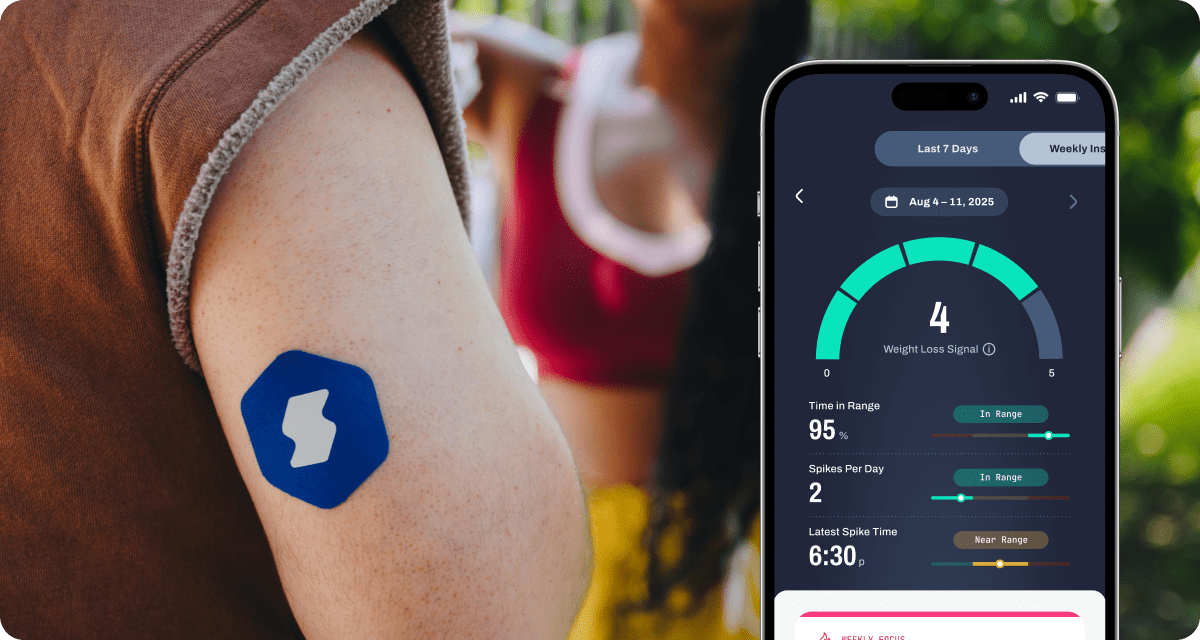
Signos gives you a continuous view of your glucose patterns, surfacing subtle signals (like post-meal spikes, overnight elevations, or morning baseline shifts) that may point to underlying metabolic stress. Through real-time nudges and personalized insights, Signos helps you respond before these patterns compound into longer-term respiratory strain or inflammation.
Your Weekly Insights report also connects glucose data to broader wellness factors like sleep, movement, and stress, allowing you to see how your metabolic health may be affecting your breathing or energy levels. For example:
- Are your higher morning glucose levels linked to poor sleep or evening snacking?
- Do you notice more shallow breathing or fatigue on days your glucose stays elevated?
- Are your post-meal spikes more frequent after sedentary days?
To go deeper, try a few in-app experiments:
- Breath + Glucose Experiment: Track your glucose before and after a short breathwork session or mindful breathing exercise to see how stress modulation affects your readings.
- Inflammation Insight Test: Compare your average glucose levels during weeks of high-processed food intake versus when you focus on anti-inflammatory, fiber-rich meals (like leafy greens, berries, and omega-3s).
- Sleep + Recovery Test: Use Signos’ overnight glucose data to observe whether improved sleep routines (earlier bedtime, cooler room) help flatten your nighttime glucose curve and improve next-day energy or breathing ease.
By connecting these patterns, Signos helps you translate real-time metabolic data into insights about how your lifestyle choices may be influencing your respiratory resilience and overall metabolic health.
The Bottom Line
Your glucose data can reveal more than diabetes risk; it offers a window into metabolic influences behind lung health. By using this information to make targeted lifestyle adjustments, Signos can help you turn actionable insights into protective measures for both your cardiovascular and respiratory systems.
Embracing nutrition, consistent movement, good sleep, and stress resilience empowers you not only to support your metabolism but also to give your lungs a healthier, more resilient foundation.
Learn More With Signos’ Expert Advice
Signos can help you improve your health by making sense of your glucose patterns in the context of broader wellness. Visit the Signos blog to learn more about how glucose levels influence your long-term health.
Topics discussed in this article:
References
- Baffi, C. W., Wood, L., Winnica, D., Strollo, P. J., Jr, Gladwin, M. T., Que, L. G., & Holguin, F. (2016). Metabolic Syndrome and the Lung. Chest, 149(6), 1525–1534. https://doi.org/10.1016/j.chest.2015.12.034
- Sagun, G., Gedik, C., Ekiz, E., Karagoz, E., Takir, M., & Oguz, A. (2015). The relation between insulin resistance and lung function: a cross sectional study. BMC pulmonary medicine, 15, 139. https://doi.org/10.1186/s12890-015-0125-9
- Peters, M. C., Schiebler, M. L., Cardet, J. C., Johansson, M. W., Sorkness, R., DeBoer, M. D., Bleecker, E. R., Meyers, D. A., Castro, M., Sumino, K., Erzurum, S. C., Tattersall, M. C., Zein, J. G., Hastie, A. T., Moore, W., Levy, B. D., Israel, E., Phillips, B. R., Mauger, D. T., Wenzel, S. E., … National Heart, Lung, and Blood Institute Severe Asthma Research Program-3 (2022). The Impact of Insulin Resistance on Loss of Lung Function and Response to Treatment in Asthma. American journal of respiratory and critical care medicine, 206(9), 1096–1106. https://doi.org/10.1164/rccm.202112-2745OC
- Lee, J., Park, H. K., Kwon, M. J., Ham, S. Y., Gil, H. I., Lim, S. Y., & Song, J. U. (2023). The impact of insulin resistance on the association between metabolic syndrome and lung function: the Kangbuk Samsung Health Study. Diabetology & metabolic syndrome, 15(1), 65. https://doi.org/10.1186/s13098-023-01042-9
- Singh, S., Bodas, M., Bhatraju, N. K., Pattnaik, B., Gheware, A., Parameswaran, P. K., Thompson, M., Freeman, M., Mabalirajan, U., Gosens, R., Ghosh, B., Pabelick, C., Linneberg, A., Prakash, Y. S., & Agrawal, A. (2016). Hyperinsulinemia adversely affects lung structure and function. American journal of physiology. Lung cellular and molecular physiology, 310(9), L837–L845. https://doi.org/10.1152/ajplung.00091.2015
- Lee, Y. Y., Tsao, Y. C., Yang, C. K., Chuang, C. H., Yu, W., Chen, J. C., & Li, W. C. (2020). Association between risk factors of metabolic syndrome with lung function. European journal of clinical nutrition, 74(5), 811–817. https://doi.org/10.1038/s41430-018-0369-6
- Choi, Y., Balte, P. P., Jacobs, D. R., Jr, London, S. J., Navas-Acien, A., Newman, A. B., O'Connor, G. T., Schwartz, J. E., Smith, B. M., White, W. B., Yende, S., & Oelsner, E. C. (2025). Associations of metabolic syndrome with lung function decline and clinical respiratory outcomes: the NHLBI Pooled Cohort Study. Eur respir j open research, 11(4), 01337-2024. https://doi.org/10.1183/23120541.01337-2024
- Kiran, Z., Majeed, N., & Zuberi, B. F. (2015). Comparison of frequency of insulin resistance in patients with chronic obstructive pulmonary disease with normal controls. Pakistan journal of medical sciences, 31(6), 1506–1510. https://doi.org/10.12669/pjms.316.7983
- Rakotoarisoa, L., Weiss, L., Lefebvre, F., Porzio, M., Renaud-Picard, B., Ravoninjatovo, B., Abely, M., Danner-Boucher, I., Dubois, S., Troussier, F., Prevotat, A., Rault, G., Kessler, R., & Kessler, L. (2024). Early glucose abnormalities revealed by continuous glucose monitoring associate with lung function decline in cystic fibrosis: A five-year prospective study. Journal of diabetes and its complications, 38(4), 108703. https://doi.org/10.1016/j.jdiacomp.2024.108703
- Yang, Y., Zhao, L. H., Li, D. D., Xu, F., Wang, X. H., Lu, C. F., Wang, C. H., Yu, C., Zhang, X. L., Ning, L. Y., Wang, X. Q., Su, J. B., & Wang, L. H. (2021). Association of sleep quality with glycemic variability assessed by flash glucose monitoring in patients with type 2 diabetes. Diabetology & metabolic syndrome, 13(1), 102. https://doi.org/10.1186/s13098-021-00720-w
- Choe, E. K., Kang, H. Y., Lee, Y., Choi, S. H., Kim, H. J., & Kim, J. S. (2018). The longitudinal association between changes in lung function and changes in abdominal visceral obesity in Korean non-smokers. PloS one, 13(2), e0193516. https://doi.org/10.1371/journal.pone.0193516
- Lee, Y. Y., Tsao, Y. C., Yang, C. K., Chuang, C. H., Yu, W., Chen, J. C., & Li, W. C. (2020). Association between risk factors of metabolic syndrome with lung function. European journal of clinical nutrition, 74(5), 811–817. https://doi.org/10.1038/s41430-018-0369-6
- Grosso, G., Laudisio, D., Frias-Toral, E., Barrea, L., Muscogiuri, G., Savastano, S., & Colao, A. (2022). Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients, 14(6), 1137. https://doi.org/10.3390/nu14061137
- Choi, H. S., Lee, S. W., Kim, J. T., & Lee, H. K. (2020). The Association between Pulmonary Functions and Incident Diabetes: Longitudinal Analysis from the Ansung Cohort in Korea. Diabetes & metabolism journal, 44(5), 699–710. https://doi.org/10.4093/dmj.2019.0109
- Sagun, G., Gedik, C., Ekiz, E., Karagoz, E., Takir, M., & Oguz, A. (2015). The relation between insulin resistance and lung function: a cross sectional study. BMC pulmonary medicine, 15, 139. https://doi.org/10.1186/s12890-015-0125-9
- Papassotiriou, I., & Shariful Islam, S. M. (2021). Adherence to Mediterranean Diet Is Associated With Lung Function in Older Adults: Data From the Health and Retirement Study. Journal of the American College of Nutrition, 40(2), 119–124. https://doi.org/10.1080/07315724.2020.1740114
- Yaribeygi, H., Atkin, S. L., Simental-Mendía, L. E., & Sahebkar, A. (2019). Molecular mechanisms by which aerobic exercise induces insulin sensitivity. Journal of cellular physiology, 234(8), 12385–12392. https://doi.org/10.1002/jcp.28066
- Angane, E. Y., & Navare, A. A. (2017). Effects of aerobic exercise on pulmonary function tests in healthy adults. International J Research in Medical Sciences, 4(6), 2059-63.
- Yadav, A., Singh, S., Singh, K., & Pai, P. (2015). Effect of yoga regimen on lung functions including diffusion capacity in coronary artery disease patients: A randomized controlled study. International journal of yoga, 8(1), 62–67. https://doi.org/10.4103/0973-6131.146067
- Sondrup, N., Termannsen, A. D., Eriksen, J. N., Hjorth, M. F., Færch, K., Klingenberg, L., & Quist, J. S. (2022). Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep medicine reviews, 62, 101594. https://doi.org/10.1016/j.smrv.2022.101594
- Yaribeygi, H., Maleki, M., Butler, A. E., Jamialahmadi, T., & Sahebkar, A. (2022). Molecular mechanisms linking stress and insulin resistance. EXCLI journal, 21, 317–334. https://doi.org/10.17179/excli2021-4382




.svg)










.svg)
.svg)
.svg)
.svg)
.svg)
.svg)
.svg)
