Key Takeaways
- Seasonal changes can disrupt gut health and metabolism, increasing the risk of metabolic stress and dysfunction.
- Postbiotics, bioactive compounds produced by bacteria, may support gut health by influencing glucose levels, reducing inflammation, and strengthening the gut barrier.
- Incorporating fermented foods, prebiotics, and postbiotic supplements into your diet can help maintain metabolic resilience and gut health throughout seasonal shifts.
{{mid-cta}}
Summer travel disrupts routines. Autumn invites immune challenges. Winter indulgence leaves you sluggish. These shifts don’t just cause temporary discomfort; they disrupt the gut microbiota, weaken the intestinal barrier, and trigger metabolic stress.
Enter postbiotics: not live bacteria, but the bioactive metabolites microbes leave behind after fermentation or cell death.1 Unlike probiotics, postbiotics don’t need refrigeration, don’t die in transit, and don’t depend on colonizing your gut to be effective. They’re shelf-stable resilience in a capsule or on your plate.
Emerging research suggests that postbiotics may:2
- Support glucose homeostasis by improving insulin sensitivity
- Influence lipid metabolism and fat storage in adipose tissue
- Modulate immune responses through cytokine signaling
- Strengthen the intestinal barrier function to keep pathogens out
- Reduce chronic, pro-inflammatory activity linked to obesity and metabolic disorders
In other words, they help maintain metabolic homeostasis even when life throws your gut off balance.
Postbiotics vs. Probiotics: What’s the Difference?

Gut health isn’t just a buzzword; it’s a complex biochemical network where live bacteria, their fuel, and their metabolites all interact to shape human health.3 Understanding the difference between probiotics, prebiotics, and postbiotics helps clarify how each piece contributes to metabolic resilience.
Probiotics
- Live microorganisms, often lactic acid bacteria (like Lactobacillus plantarum or Lactobacillus rhamnosus) and *bifidobacterium.
- Shown in clinical trials to support digestion, immunity, and even reduce symptoms of colitis or gastrointestinal distress.
- Fragile: heat, antibiotics, or stomach acid can kill them before they colonize the intestinal microbiota.
Prebiotics
- Non-digestible carbohydrates (like inulin, resistant starch, and beta-glucans) that serve as food for gut microbes.
- When fermented, these fibers are converted by commensal gut microbes into short-chain fatty acids (SCFAs), including butyrate, propionate, and acetate.
- Found naturally in whole grains, garlic, onions, bananas, legumes, and oats.
Postbiotics
- Not live organisms, but the bioactive metabolites and cell-wall components microbes leave behind.4
- Includes: SCFAs, peptides, exopolysaccharides, bacteriocins, enzymes, lipoteichoic acid, vitamins, and antioxidant compounds.5
- Heat-stable, shelf-stable, and not reliant on colonization. That makes them a practical and consistent tool when the gut microbiome is disrupted by stress, a high-fat diet, or antibiotics.
- Effects of postbiotics documented in both in vitro and in vivo research include: antimicrobial activity, immune modulation, barrier protection, and improved metabolic pathways.6,7,8
Quick analogy:
- Probiotics = chefs.
- Prebiotics = ingredients.
- Postbiotics = the finished meal; already cooked, packed with bioactive nutrients, and ready to deliver beneficial effects without waiting for live bacteria to survive.
The Science: Postbiotics & Metabolic Support

Glucose Regulation & Energy Balance
One of the most promising areas of postbiotic research centers on glucose and energy control, critical for preventing type 2 diabetes and other metabolic diseases.8
SCFAs like butyrate and acetate:9
- Enhance insulin sensitivity in tissues.
- Influence signaling pathways that regulate glucose uptake and production in the liver.
- Promote the secretion of gut hormones that regulate appetite and satiety.
Clinical studies suggest postbiotic supplementation can lower fasting glucose and improve insulin sensitivity.9,10 In vivo animal research shows SCFAs reduce weight gain and insulin resistance triggered by a high-fat diet.9,10
Translation: postbiotics help the body maintain glucose homeostasis, steady energy without the rollercoaster spikes and crashes.
Lipid Modulation & Cardiovascular Health
The gut doesn’t just influence carbs; it also shapes how the body handles fats.
SCFAs and other microbial metabolites act on enzymes that regulate fat storage in adipose tissue.9
Postbiotics may:8,9,10
- Lower triglycerides and cholesterol.
- Reduce lipid accumulation linked to obesity and metabolic syndrome.
- Protect vascular health by lowering low-grade inflammation that drives cardiovascular dysfunction.
Some controlled trials have shown improvement in lipid panels after supplementation with inactivated bacterial strains, such as Lactobacillus acidophilus.12,13
Translation: postbiotics may help “reset” lipid metabolism, reducing risks tied to cardiovascular diseases and supporting long-term metabolic balance.12,13
Anti-Inflammatory & Immune Support
Chronic, low-grade inflammation is at the core of many metabolic disorders, from obesity to type 2 diabetes. Postbiotics appear to act like immune balancers, calming excess response while still defending against threats.4
Immunomodulatory mechanisms:12
- Reduce pro-inflammatory cytokines like TNF.
- Enhance production of anti-inflammatory peptides and signaling molecules.
- Strengthen mucosal immunity by stimulating differentiation of protective immune cells.
Barrier Function:13
- Compounds like butyrate nourish gut epithelial cells, thereby maintaining the intestinal barrier and reducing harmful permeability, also known as a “leaky gut.”
- This prevents LPS (lipopolysaccharides) and other bacterial toxins from entering the circulation, thereby reducing systemic inflammation.
Immune Signaling:12,13
- Engage toll-like receptors and macrophages to fine-tune immune responses.
- Prevent overactivation that leads to chronic inflammation while still inhibiting pathogens.
Translation: postbiotics don’t just patch up the gut; they help recalibrate the immune system and reduce the silent inflammation tied to metabolic decline.
Why Seasonal Shifts Matter for Gut Health
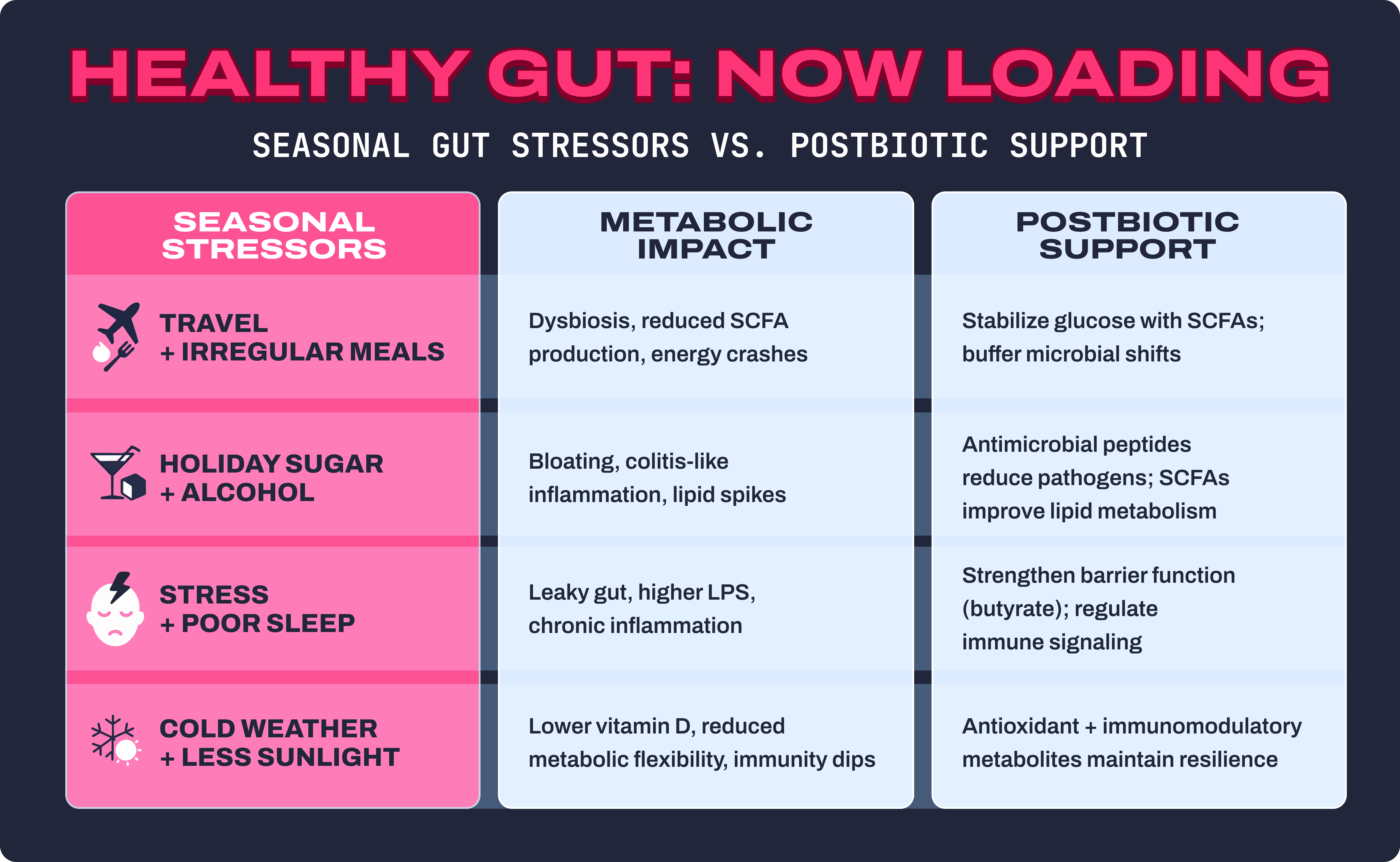
Your gut microbiome is a living, adaptive ecosystem, but it doesn’t thrive under chaos. Seasonal changes, lifestyle disruptions, and diet swings all create stressors that ripple through the gut–brain–metabolic axis. Here’s how each seasonal trigger messes with balance:
Travel + irregular meals = dysbiosis and energy crashes
Crossing time zones, skipping meals, and eating airport food can shrink microbial diversity. Gut bacteria thrive on routine; when feeding times shift, so does their rhythm of fermentation and SCFA production. That means fewer short-chain fatty acids to stabilize blood sugar, leaving you with energy crashes and carb cravings.14
Holiday sugar + alcohol = colitis, bloating, lipid spikes
High-sugar indulgences fuel pathogenic bacteria and increase gut permeability. Alcohol amplifies this effect by damaging the gut lining and altering the intestinal microbiota. The result: bloating, inflammation, and even flare-ups of colitis-like symptoms. On a metabolic level, this combination elevates triglycerides and can predispose the body to insulin resistance.14,15
Stress + poor sleep = immune dysfunction and higher LPS-driven inflammation
Seasonal stress (holidays, deadlines, cold/flu season) cranks up cortisol, which disrupts microbial balance. Sleep loss further weakens the intestinal barrier function and reduces the production of beneficial butyrate. Together, this allows bacterial fragments like LPS (lipopolysaccharides) to leak into circulation, driving systemic, pro-inflammatory cytokine release.13,16,17
Cold weather + less sunlight = decreased homeostasis and metabolic flexibility
Shorter days mean less vitamin D and less outdoor activity, both of which affect the immune system and gut microbial composition. In winter, people often shift toward a higher-fat, lower-fiber diet, which reduces the beneficial fermentation that occurs naturally. The net effect: weakened metabolic flexibility and a harder time maintaining glucose homeostasis.
Bottom line: When the gut microbiome gets thrown off, so do cravings, immunity, mood, and metabolism. Postbiotics help buffer these seasonal disruptions by delivering bioactive, antioxidant, and antimicrobial compounds that keep your gut resilient, regardless of the season.
Sources of Postbiotics
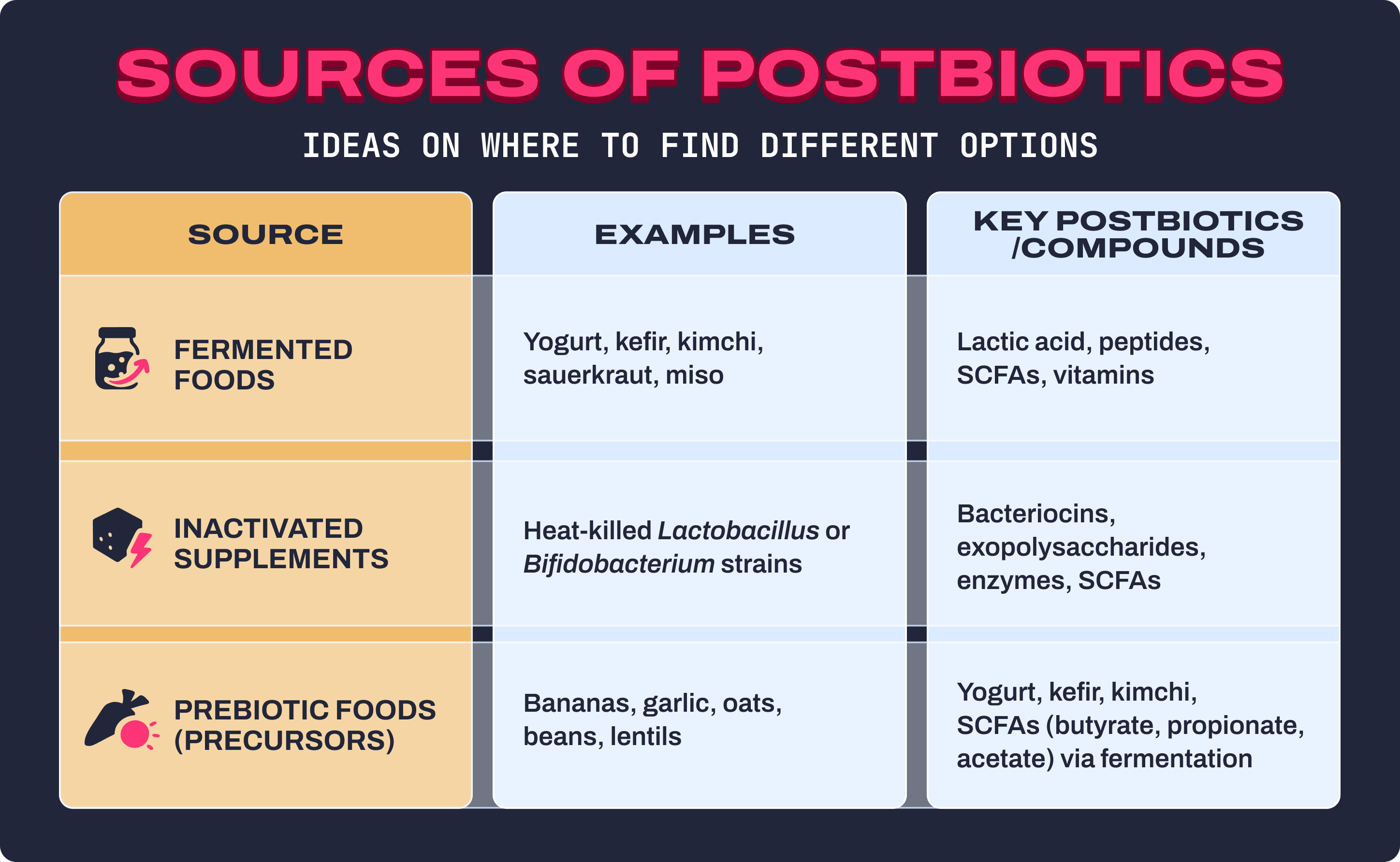
You don’t always need a supplement bottle to get postbiotics, though targeted formulas can help. Postbiotics naturally show up in fermented foods, microbial metabolites, and even heat-inactivated bacterial strains. Here’s where to find them:
Fermented Foods
- Yogurt, kefir, kimchi, sauerkraut, miso, tempeh, pickles.
- These contain both live probiotics and their metabolic byproducts: postbiotics like lactic acid, peptides, and SCFAs.
- Common strains: Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium breve.
- Benefits: improved digestion, immunomodulatory activity, antimicrobial defense, and increased bioavailability of nutrients.
Inactivated Supplements
- Heat-killed Gram-positive bacteria still deliver beneficial bioactive compounds, including bacteriocins, exopolysaccharides, enzymes, and SCFAs.18,19
- Because they aren’t live, they’re stable, safe (even for immune-compromised individuals), and don’t require refrigeration.
- Controlled trials have shown that these inactivated strains can reduce diarrhea, improve lipid metabolism, and strengthen intestinal barrier function.
Prebiotic Foods (postbiotic precursors)
- Fiber-rich foods like bananas, garlic, oats, beans, lentils, apples, asparagus, and chicory root.
- These non-digestible carbohydrates fuel microbial fermentation, which leads to the production of SCFAs, especially butyrate, propionate, and acetate.20
- This process turns everyday fiber into the postbiotic metabolites that drive many of the gut’s beneficial effects.
Pro tip: Combine all three. Eat prebiotic fibers to fuel microbes, enjoy fermented foods for natural postbiotic exposure, and use inactivated supplementation during high-stress, travel, or immune-challenged seasons for an extra buffer.
The Metabolic Playbook: Supporting Gut Health Through the Seasons

Spring
- Ramp up fermented foods before allergy season to reduce pro-inflammatory cytokines
- Add prebiotics to boost microbial proliferation and SCFA secretion
Summer
- Focus on hydration and high-fiber fruits/vegetables to sustain gut microbial diversity
- Use postbiotic supplementation to maintain gut integrity during travel
Fall
- Pair probiotic foods with postbiotic supplements for stronger immune support
- Layer in polyphenol-rich functional foods (berries, green tea) for antioxidant and antimicrobial activity21,22
Winter
- Reinforce gut resilience with inactivated bacterial supplementation and SCFA-rich dairy
- Counteract high-fat diet indulgences with fiber + fermented foods to protect against dysbiosis
Integration with Lifestyle: Beyond the Supplement

Postbiotics are powerful, but they’re only one part of the metabolic health equation. Their impact is amplified (or undermined) by everyday lifestyle choices that shape the gut–brain–immune axis. To get the most from postbiotics, integrate them into a foundation of healthy habits:
Balanced macronutrients
- A mix of proteins, complex carbohydrates, and healthy fats provides steady glucose release while feeding beneficial microbes.
- Complex carbs (like oats, lentils, and vegetables) supply prebiotic fibers, which gut bacteria ferment into short-chain fatty acids (SCFAs), the same metabolites many postbiotics deliver directly.
- Pairing protein and healthy fats with fiber helps blunt glucose spikes, reduces cravings, and creates a nutrient-rich environment where postbiotics can thrive.
Movement
- Regular physical activity boosts gut motility (keeping digestion on track) and increases microbial diversity.
- Exercise influences SCFA production, particularly butyrate, which supports energy metabolism and insulin sensitivity.
- Even moderate activity (like brisk walking or resistance training) has been shown to enhance metabolic flexibility, helping the body shift between glucose and fat as fuel.
Sleep & stress
- Poor sleep and chronic stress elevate cortisol, which disrupts the gut–brain axis and increases gut permeability.
- Lack of restorative sleep also lowers microbial diversity and reduces beneficial postbiotic metabolites like butyrate.
- Prioritizing 7–9 hours of consistent, high-quality sleep and using stress-buffering practices (meditation, breathwork, time in nature) helps keep the microbiome resilient.
Antibiotics (use wisely)
- While lifesaving when needed, antibiotics are a leading disruptor of gut microbial homeostasis.
- Overuse can wipe out beneficial strains, reduce microbial diversity, and leave behind dysbiosis that fuels inflammation.
- Postbiotics may play a role in recovery, as they don’t require live colonization and can help rebalance the immune response.
Takeaway: Postbiotics work best as part of a comprehensive whole-system approach, where diet, movement, stress, and sleep all interact with the microbiome to influence metabolic outcomes.
Safety & Quality

One of the key advantages of postbiotics: they’re considered safe and stable across a wide range of populations, even those who may be vulnerable to complications from live bacteria (such as immunocompromised individuals, infants, or the elderly).23
Safety profile
- Unlike probiotics, postbiotics don’t require survival through the digestive tract or colonization of the gut.
- Controlled human trials report promising results with minimal side effects.
- Because they are heat-stable and shelf-stable, postbiotics avoid the fragility issues that can undermine probiotic effectiveness.
What to look for in quality postbiotics
- Clearly listed bioactive compounds: Products should specify whether they contain SCFAs, peptides, exopolysaccharides, or other functional metabolites.
- Transparent labeling of strains: If inactivated bacteria are used, the label should name the strain (e.g., Lactobacillus plantarum [heat-inactivated]) rather than vague “fermentate” claims.
- Third-party testing: Independent lab verification ensures purity, potency, and absence of contaminants.
- Evidence-based formulations: Look for citations to clinical trials or mechanistic studies backing up claims.
Takeaway: While more large-scale clinical studies are needed, current evidence suggests that postbiotics are safe, effective, and practical for long-term use, especially when sourced from transparent, third-party tested brands.
The Bottom Line

Seasonal stress (travel, holidays, weather shifts) creates ripple effects in the gut. That can trigger dysbiosis, metabolic dysfunction, immune stress, and even weight challenges tied to obesity and insulin resistance.
Postbiotics aren’t magic. However, when combined with fiber, fermented foods, smart supplementation, quality sleep, and regular movement, they deliver beneficial effects that stabilize your gut ecosystem, reinforce barrier function, and reduce inflammation.
Think of them as metabolic insurance: bioactive allies that help you stay resilient, even when the season throws your gut off balance.
Learn More With Signos’ Expert Advice
Use data, not guesswork. Continuous glucose monitors (CGMs) track glucose levels, allowing you to see how sleep, stress, meal timing, and food pairings impact cravings.
Signos’ expert-written blog provides additional opportunities to learn more about how CGMs can help you rewrite your appetite to break a food rut.
Topics discussed in this article:
References
- Calvanese, C. M., Villani, F., Ercolini, D., & De Filippis, F. (2025). Postbiotics versus probiotics: Possible new allies for human health. Food res international (Ottawa, Ont.), 217, 116869. https://doi.org/10.1016/j.foodres.2025.116869
- Park, M., Joung, M., Park, J. H., Ha, S. K., & Park, H. Y. (2022). Role of Postbiotics in Diet-Induced Metabolic Disorders. Nutrients, 14(18), 3701. https://doi.org/10.3390/nu14183701
- https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/
- Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., Sanders, M. E., Shamir, R., Swann, J. R., Szajewska, H., & Vinderola, G. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature reviews. Gastroenterol & hepatology, 18(9), 649–667. https://doi.org/10.1038/s41575-021-00440-6
- Ma, L., Tu, H., & Chen, T. (2023). Postbiotics in Human Health: A Narrative Review. Nutrients, 15(2), 291. https://doi.org/10.3390/nu15020291
- Pham, N. H. T., Joglekar, M. V., Wong, W. K. M., Nassif, N. T., Simpson, A. M., & Hardikar, A. A. (2024). Short-chain fatty acids and insulin sensitivity: a systematic review and meta-analysis. Nutrition reviews, 82(2), 193–209. https://doi.org/10.1093/nutrit/nuad042
- He, J., Zhang, P., Shen, L., Niu, L., Tan, Y., Chen, L., Zhao, Y., Bai, L., Hao, X., Li, X., Zhang, S., & Zhu, L. (2020). Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. International journal of molecular sciences, 21(17), 6356. https://doi.org/10.3390/ijms21176356
- Park, S. J., Sharma, A., & Lee, H. J. (2023). Postbiotics against Obesity: Perception and Overview Based on Pre-Clinical and Clinical Studies. International journal of molecular sciences, 24(7), 6414. https://doi.org/10.3390/ijms24076414
- van Deuren, T., Blaak, E. E., & Canfora, E. E. (2022). Butyrate to combat obesity and obesity-associated metabolic disorders: Current status and future implications for therapeutic use. Obesity reviews : an official journal of the International Association for the Study of Obesity, 23(10), e13498. https://doi.org/10.1111/obr.13498
- Kumar, A., Green, K. M., & Rawat, M. (2024). A Comprehensive Overview of Postbiotics with a Special Focus on Discovery Techniques and Clinical Applications. Foods (Basel, Switzerland), 13(18), 2937. https://doi.org/10.3390/foods13182937
- Liu, T., Sun, Z., Yang, Z., & Qiao, X. (2023). Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 162, 114586. https://doi.org/10.1016/j.biopha.2023.114586
- Hodgkinson, K., El Abbar, F., Dobranowski, P., Manoogian, J., Butcher, J., Figeys, D., Mack, D., & Stintzi, A. (2023). Butyrate's role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clinical nutrition (Edinburgh, Scotland), 42(2), 61–75. https://doi.org/10.1016/j.clnu.2022.10.024
- Wu, H., & Ballantyne, C. M. (2020). Metabolic Inflammation and Insulin Resistance in Obesity. Circulation research, 126(11), 1549–1564. https://doi.org/10.1161/CIRCRESAHA.119.315896
- Thursby, E., & Juge, N. (2017). Introduction to the human gut microbiota. The Biochemical journal, 474(11), 1823–1836. https://doi.org/10.1042/BCJ20160510
- Hrncir T. (2022). Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms, 10(3), 578. https://doi.org/10.3390/microorganisms10030578hj
- Aja, E., Zeng, A., Gray, W., Connelley, K., Chaganti, A., & Jacobs, J. P. (2025). Health Effects and Therapeutic Potential of the Gut Microbe Akkermansia muciniphila. Nutrients, 17(3), 562. https://doi.org/10.3390/nu17030562
- Zhang, Y., Pu, F., Cheng, R., Guo, J., Shen, X., Wang, S., Zhu, H., Zhang, X., Cheng, G., Li, M., & He, F. (2020). Effect of heat-inactivated Lactobacillus paracasei N1115 on microbiota and gut-brain axis related molecules. Bioscience of microbiota, food and health, 39(3), 89–99. https://doi.org/10.12938/bmfh.2019-025
- Guo, S., Ma, T., Kwok, L. Y., Quan, K., Li, B., Wang, H., Zhang, H., Menghe, B., & Chen, Y. (2024). Effects of postbiotics on chronic diarrhea in young adults: a randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles. Gut microbes, 16(1), 2395092. https://doi.org/10.1080/19490976.2024.2395092
- Lima, M., & Paulino, L. C. (2024). Oral Postbiotics as a Therapeutic Strategy for Atopic Dermatitis: A Systematic Review of Randomized Controlled Trials. Journal of the American Nutrition Association, 43(2), 139–146. https://doi.org/10.1080/27697061.2023.2232021
- Singh, R. P., & Bhardwaj, A. (2023). β-glucans: a potential source for maintaining gut microbiota and the immune system. Frontiers in nutrition, 10, 1143682. https://doi.org/10.3389/fnut.2023.1143682
- Kuerec, A. H., Lim, X. K., Khoo, A. L., Sandalova, E., Guan, L., Feng, L., & Maier, A. B. (2024). Targeting aging with urolithin A in humans: A systematic review. Ageing research reviews, 100, 102406. https://doi.org/10.1016/j.arr.2024.102406
- Zhao, H., Song, G., Zhu, H., Qian, H., Pan, X., Song, X., Xie, Y., & Liu, C. (2023). Pharmacological Effects of Urolithin A and Its Role in Muscle Health and Performance: Current Knowledge and Prospects. Nutrients, 15(20), 4441. https://doi.org/10.3390/nu15204441
- Isaac-Bamgboye, F. J., Mgbechidinma, C. L., Onyeaka, H., Isaac-Bamgboye, I. T., & Chukwugozie, D. C. (2024). Exploring the Potential of Postbiotics for Food Safety and Human Health Improvement. Journal of nutrition and metabolism, 2024, 1868161. https://doi.org/10.1155/2024/186816




.svg)










.svg)
.svg)
.svg)
.svg)
.svg)
.svg)
.svg)
